Updated Coronavirus Disease (COVID-19) & Other Respiratory Illness Response
The Hamre Center is offering guidance to students and those supporting students, though much of the best practice is the same for everyone.
When you may have a respiratory virus…
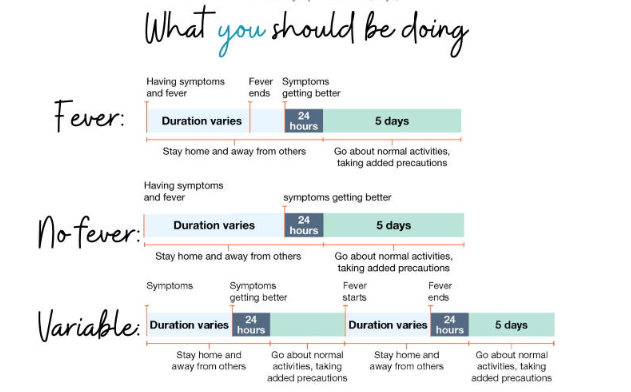
Stay home and away from others (including people you live with who are not sick) if you have respiratory virus symptoms that aren’t better explained by another cause. These symptoms can include fever, chills, fatigue, cough, runny nose, and headache, among others.
- You can go back to your normal activities when, for at least 24 hours, both are true:
- Your symptoms are getting better overall, and
- You have not had a fever (and are not using fever-reducing medication).
- When you go back to your normal activities, take added precaution over the next 5 days, such as taking additional steps for cleaner air, hygiene, masks, physical distancing, and/or testing when you will be around other people indoors.
- Keep in mind that you may still be able to spread the virus that made you sick, even if you are feeling better. You are likely to be less contagious at this time, depending on factors like how long you were sick or how sick you were.
- If you develop a fever or you start to feel worse after you have gone back to normal activities, stay home and away from others again until, for at least 24 hours, both are true: your symptoms are improving overall, and you have not had a fever (and are not using fever-reducing medication). Then take added precaution for the next 5 days.
Graphic: Dr. Katelyn Jetelina, Your Local Epidemiologist March 2024
When you have a respiratory virus infection, you can spread it to others. How long someone can spread the virus depends on different factors, including how sick they are (severity) and how long their illness lasts (duration). This is not the same for everyone.
Students: You can contact the Hamre Center, 651-696-6275 or [email protected], during business hours to make an appointment. Call the Access Nurse line (651-696-6275, option 3)* for 24/7 medical advice. If you are experiencing severe symptoms and want to be seen urgently, see this list of urgent and emergency care medical resources.
We encourage faculty, coaches, and supervisors to be supportive in allowing those with COVID to isolate until they test negative (up to 10 full days); allowing them to get well and to minimize their chances of infecting others.
Residential students
While in isolation, you can use your campus meal plan and order a meal to pick up to go, you can use the Isolation Meal Order Form located on Bon Appetit’s website. You can subscribe to the menu mail Macalester College – Fresh. Local. Delicious. (cafebonappetit.com) to receive the menu in your inbox. You are able to pick up your meal yourself as long as you wear a high quality mask indoors, and you can also designate a friend to pick it up too.
Please note: orders need to be submitted by 7 p.m. the night before your selected pick-up date.
Testing
Macalester no longer has access to bulk free at-home tests through the Minnesota Department of Health; however MDH continues to offer free, rapid at-home tests to individuals (4 kits/8 tests per household per calendar month) while supplies last. Tests can be ordered from sayyeshometest. Residential students please include your SPO in your address (in the apt line) to request tests. Non-residential students should request tests sent to their off-campus address. We encourage all campus community members to have COVID tests on hand.
Masking
Wearing high-quality, N95 or KN95 masks continues to be one of the most effective prevention steps you can take. Free masks are available inside and outside the Hamre Center, at the front desk of the library, and through the food pantry. We encourage our campus to continue to welcome masking.
Hamre center has transitioned our masking policy to be OPTIONAL within our clinical spaces. However, face masks will still be required for anyone with respiratory symptoms (Cough, sore throat, or fever) while in the Hamre Center.
If you prefer to wear masks, and/or that your provider wears one as well when providing care to you, we will respect that request 100%. Please feel comfortable asking for this accommodation-we want everyone to feel safe and affirmed within our locations. Please do not hesitate to contact our office with any questions or concerns or if you are needing additional accommodations.
If you’d like to post a sign in your individual space requesting others to mask in that space
Vaccinations
Vaccination continues to be highly effective in preventing severe disease and hospitalization in most populations. Macalester highly recommends staying up to date on your COVID vaccinations per CDC guidelines, but does not currently require that you do so. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html
COVID-19 vaccines will no longer be free through the federal government.
Hamre will continue to provide information on where to access vaccinations in the community, including local Medicaid-eligible sites. The Hamre Center will offer the COVID-19 vaccine on campus this fall.
We encourage faculty, coaches, and supervisors to be supportive in allowing people who have reactions to the vaccinations to take the time they need to recover.
Isolate-in-place policy for residential students
Since August 2022, along with the majority of institutions of higher education, Macalester moved to an isolate-in-place plan for COVID. This is parallel to how most other respiratory diseases, such as influenza, have been handled on campuses. A roommate of a student who has tested positive for COVID is free to make their own decisions about where to stay.
Students who are immunocompromised or may have other reasons to request an accommodation can request to be moved to temporary Covid Isolation housing through the Center for Disability Resources if their roommate tests positive for COVID.
Additional questions about COVID can be sent to [email protected].
Updated April 2024